CD28 humanized mouse model for efficacy and safety assessment of CD28-targeting therapies
CD28-targeting therapies
Abstract
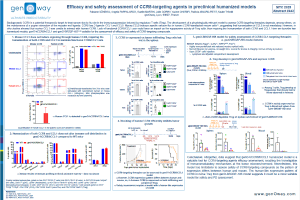
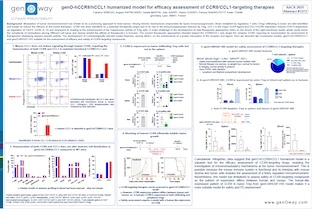
Agonist antibodies targeting CD28 have proven to be effective against cancer, but also faced challenges due to severe adverse events triggered by its activation. Human (hCD28) and mouse CD28 (mCD28) have different signaling responses, with CD28 ligands and superagonists inducing pro-inflammatory cytokines upon stimulation in absence of TCR ligation in humans, but not in mice. Expression of CD28i amplifier isoform, which is thought to enhanced the production of cytokines in humans, could partially explain this difference, as it is not expressed in mice. In addition, evidence suggests that the different signaling between hCD28 and mCD28 relies on one amino acid change in the intracellular domain (ICD)[1]. Herein, we describe a CD28 humanized mouse model for assessment of CD28-targeting agents. To improve translatability, we decided to keep the expression of both canonical and CD28i human isoforms to avoid undermining the biological effects of the testing agents. Although keeping the human ICD could favor the evaluation of cytokine production and therefore the safety of the test agents, we decided to keep the mouse ICD to enable a proper interaction of CD28 with its signaling partners, allowing a physiological stimulation of CD28 in efficacy studies. The hCD28 model expresses a chimeric protein, with the whole human extracellular domain, and mouse intracellular domain. Human CD28 is expressed at physiological levels on T cells, and these cells can be activated and proliferate in response to hCD28 ligands and agonist antibodies. Tumor growth inhibition and complete tumor regressions are achieved in a MC38-TAA colorectal cancer model following prophylactic treatment with CD28-TAA bispecific antibody in combination with anti-PD-1. Extension of this study and re-challenged of the tumor-free surviving animals shows that re-challenged hCD28 mice have enhanced survival compared to naïve hCD28 mice inoculated with MC38-TAA, suggesting tumor immunological memory. Furthermore, TGN1412 administration in CD28 mice induces cytokine release, and a slightly body weight loss, but no major change in body temperature. Although the model does not fully reproduce the ability of human CD28 to produce cytokines upon activation due to the mouse ICD, the expression of the amplifier isoform CD28i might contribute to the response to TGN1412. Altogether, data suggest that hCD28 model enables assessment of efficacy and safety of CD28-targeting agents. The hCD28 model was intercrossed with CD3 humanized models to enable assessment of combination therapies and bispecific antibodies targeting both CD3 and CD28.
[1] Porciello N, Grazioli P, Campese AF, et al. A non-conserved amino acid variant regulates differential signalling between human and mouse CD28. Nat Commun 2018; 9:1-16

Scientific excellence
From model design to experimental results
Featured in 600+ scientific articles

Collaborative approach
Collaboration with 17 Top Pharmas,
170+ Biotechs and 380+ Academic Institutions

Robust validation data on catalog models
Generated with biopharma partners and in-house

Innovative technologies
and guaranteed freedom to operate

Easy access to models
Models with certified health status from professional breeders in US and Europe