Part 2: BRGSF, a new immunodeficient model for immuno-oncology studies
As discussed in our previous commentary, mice are the experimental models of election in preclinical research, as they represent an affordable, rapidly reproducing and easily maintained mammalian model to study disease etiology and therapeutic assessment.1,2 Syngeneic tumor transplantation and genetically engineered mouse models have offered tremendous insight in the basic mechanisms of immune regulation. However, for many years, substantial genetic and genomic differences between humans and mice have hampered the translatability of experimental findings in infectious and immune-related diseases, very often leading to clinical trial failures.3,4
In order to bridge this gap and enhance the transfer of knowledge from in vitro and mouse-based investigations toward clinical applications, scientists have generated a wide variety of immunodeficient models such as SCID, NUDE and Rag-deficient mice engrafted either subcutaneously or orthotopically with cell-derived xenografts (CDXs) or patient-derived xenografts (PDXs).5,6
NUDE mice were mainly used to investigate cancer initiation and progression, as these animals could be easily engrafted subcutaneously due to the absence of fur. SCID and Rag-deficient mice, on the other hand, were the perfect hosts to study blood cancers such as lymphoma, myeloma, and leukemia, as they are more immunodeficient (i.e., they both lack T and B cells) than NUDE mice.5 All these models, however, still possessed residual murine immune cells and, therefore, could not be used for long-term xenograft studies. Moreover, the lack of functional immune system was of major concern for a proper understanding of the pathophysiology of tumor development, escape mechanism to immune system, and assessment of immunotherapies enabling the development of effective anti-tumor response.2,7
For all these reasons, scientists developed a new generation of highly immunodeficient mice by backcrossing SCID and Rag-deficient mice into non-obese diabetic (NOD) or Balb/c mice. The resulting strains, NOG (NOD/Shi-scid IL-2rγnull), NSG (NOD-scid IL-2rγ−/−) and BRGS (Balb/c Rag2tm1Fwa IL-2rγtm1Cgn SirpαNOD), carry multiple genetic defects, including mutations in i) IL‑2 receptor common γ-chain (IL-2rγ), leading to profound NK cells deficiency, ii) Prkdc (NOG and NSG) or Rag2 (BRGS), causing a depletion in B and T cells, and iii) NOD-specific polymorphic Sirpα, resulting in a reduced phagocytosis of human CD47+ cells by murine macrophages (i.e., ‘don’t eat me’ signal).8–11
The NOG and NSG were largely used in PDX studies, as they sustain better engraftments of human tissues compared to NUDE and SCID mice. However, due to the mutations in Prkdc, these strains show the SCID side effect of high sensitivity to radiation, T-cell leakage, and increased incidence of thymic lymphoma formation; as such, they cannot be used to predict clinical response to certain anticancer drugs, or for long-term transplantation studies.12,13 The BRGS solves both these problems because it does not carry the Prkdc mutation and, therefore, it does not possess the SCID phenotype observed in the NSG and NOG strain.9,10
The BRGS has been recently improved by a team of scientists at the Pasteur Institute, by backcrossing it into the BRGF (BALB/c Rag2tm1Fwa Il2rγtm1Cgn Flt3tm1lrl) strain. The resultant BRGSF (BALB/c Rag2tm1Fwa Il2rγtm1Cgn SirpαNOD Flt3tm1lrl) is highly immunodeficient, because besides carrying the genetic defects of its parental strains, it possesses a deficiency in the fetal liver kinase-2 (Flk2), which regulates the development of the myeloid compartment.14–16
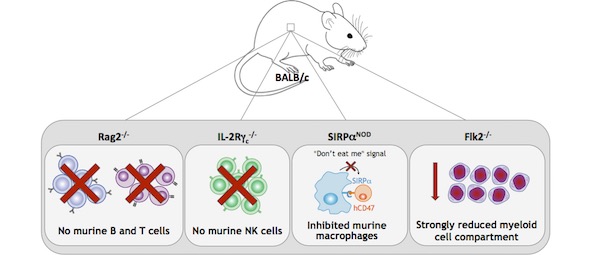
For all these reasons, the BRGSF mouse model represents a valuable tool for vaccine development, efficacy and safety of chimeric antigen receptor (CAR) T cell therapy, and myeloid compartment development studies. Moreover, as it is highly permissive to human hematopoietic cells engraftment, BRGSF serves as the optimal model to generate human immune system (HIS) mice to study and predict human immune responses in vivo.8,14–16
See also:
Part 1: Toward a better effective preclinical model in immuno-oncology
Part 3: BRGSF-HIS, a new human immune system mouse model for immuno-oncology studies
Part 4: Humanized immune checkpoint mouse models for immuno-oncology studies
Part 5: HSA/hFcRn, a powerful model for your PK/PD studies
References:
- Wagar, L. E.; DiFazio, R. M.; Davis, M. M. Advanced Model Systems and Tools for Basic and Translational Human Immunology. Genome Med. 2018, 10 (1), 73. https://doi.org/10.1186/s13073-018-0584-8.
- Wege, A. K. Humanized Mouse Models for the Preclinical Assessment of Cancer Immunotherapy. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2018, 32 (3), 245–266. https://doi.org/10.1007/s40259-018-0275-4.
- Bedognetti, D.; Ceccarelli, M.; Galluzzi, L.; Lu, R.; Palucka, K.; Samayoa, J.; Spranger, S.; Warren, S.; Wong, K.-K.; Ziv, E.; et al. Toward a Comprehensive View of Cancer Immune Responsiveness: A Synopsis from the SITC Workshop. J. Immunother. Cancer 2019, 7 (1), 131. https://doi.org/10.1186/s40425-019-0602-4.
- Attarwala, H. TGN1412: From Discovery to Disaster. J. Young Pharm. 2010, 2 (3), 332–336. https://doi.org/10.4103/0975-1483.66810.
- Allen, T. M.; Brehm, M. A.; Bridges, S.; Ferguson, S.; Kumar, P.; Mirochnitchenko, O.; Palucka, K.; Pelanda, R.; Sanders-Beer, B.; Shultz, L. D.; et al. Humanized Immune System Mouse Models: Progress, Challenges and Opportunities. Nat. Immunol. 2019, 20 (7), 770–774. https://doi.org/10.1038/s41590-019-0416-z.
- Yong, K. S. M.; Her, Z.; Chen, Q. Humanized Mice as Unique Tools for Human-Specific Studies. Arch. Immunol. Ther. Exp. (Warsz.) 2018, 66 (4), 245–266. https://doi.org/10.1007/s00005-018-0506-x.
- Perlman, R. L. Mouse Models of Human Disease: An Evolutionary Perspective. Evol. Med. Public Health 2016, 2016 (1), 170–176. https://doi.org/10.1093/emph/eow014.
- Simpson, J. A.; Brown, M. E. Making HIS Mice More Human‐like. J. Leukoc. Biol. 2020, 107 (1), 9–10. https://doi.org/10.1002/JLB.5CE1019-262R.
- Yamauchi, T.; Takenaka, K.; Urata, S.; Shima, T.; Kikushige, Y.; Tokuyama, T.; Iwamoto, C.; Nishihara, M.; Iwasaki, H.; Miyamoto, T.; et al. Polymorphic Sirpα Is the Genetic Determinant for NOD-Based Mouse Lines to Achieve Efficient Human Cell Engraftment. Blood 2013, 121 (8), 1316–1325. https://doi.org/10.1182/blood-2012-06-440354.
- Lopez-Lastra, S.; Di Santo, J. P. Modeling Natural Killer Cell Targeted Immunotherapies. Front. Immunol. 2017, 8. https://doi.org/10.3389/fimmu.2017.00370.
- Zitvogel, L.; Pitt, J. M.; Daillère, R.; Smyth, M. J.; Kroemer, G. Mouse Models in Oncoimmunology. Nat. Rev. Cancer 2016, 16 (12), 759–773. https://doi.org/10.1038/nrc.2016.91.
- Pearson, T.; Shultz, L. D.; Miller, D.; King, M.; Laning, J.; Fodor, W.; Cuthbert, A.; Burzenski, L.; Gott, B.; Lyons, B.; et al. Non-Obese Diabetic-Recombination Activating Gene-1 (NOD- Rag 1 null ) Interleukin (IL)-2 Receptor Common Gamma Chain ( IL 2 Rγ null ) Null Mice: A Radioresistant Model for Human Lymphohaematopoietic Engraftment. Clin. Exp. Immunol. 2008, 154 (2), 270–284. https://doi.org/10.1111/j.1365-2249.2008.03753.x.
- Fulop, G. M.; Phillips, R. A. The Scid Mutation in Mice Causes a General Defect in DNA Repair. Nature 1990, 347 (6292), 479–482. https://doi.org/10.1038/347479a0.
- Lopez-Lastra, S.; Masse-Ranson, G.; Fiquet, O.; Darche, S.; Serafini, N.; Li, Y.; Dusséaux, M.; Strick-Marchand, H.; Di Santo, J. P. A Functional DC Cross Talk Promotes Human ILC Homeostasis in Humanized Mice. Blood Adv. 2017, 1 (10), 601–614. doi.org/10.1182/bloodadvances.2017004358
- Li, Y.; Mention, J.-J.; Court, N.; Masse-Ranson, G.; Toubert, A.; Spits, H.; Legrand, N.; Corcuff, E.; Strick-Marchand, H.; Di Santo, J. P. A Novel Flt3-Deficient HIS Mouse Model with Selective Enhancement of Human DC Development. Eur. J. Immunol. 2016, 46 (5), 1291–1299. https://doi.org/10.1002/eji.201546132.
- Legrand, N.; Huntington, N. D.; Nagasawa, M.; Bakker, A. Q.; Schotte, R.; Strick-Marchand, H.; de Geus, S. J.; Pouw, S. M.; Böhne, M.; Voordouw, A.; et al. Functional CD47/Signal Regulatory Protein Alpha (SIRPα) Interaction Is Required for Optimal Human T- and Natural Killer- (NK) Cell Homeostasis in Vivo. Proc. Natl. Acad. Sci. 2011, 108 (32), 13224–13229. https://doi.org/10.1073/pnas.1101398108.
Scientific excellence
From model design to experimental results
Tailor-made solutions adapted to scientific questions
Robust validation data on catalog models
Comprehensive dataset package
Generated with biopharma partners and in-houseCustomer care
Scientific follow-up and advice along the project
Collaborative approach for problem solving and development of innovative models
Easy and fast access to models
Breeding facilities in US and Europe
Certified health status from professional breeders


