Part 1: Toward a better effective preclinical model in immuno-oncology
Animal research has been extensively used in oncology and onco-pharmacology to understand the mechanisms that underpin cancer development, and to design effective tumor treatments.(1) However, the challenges along the path of converting results obtained at the bench into tangible clinical endpoints are numerous and formidable, including the choice of the right model to answer precise immunological questions.(2) Organisms such as the budding yeast Saccharomyces cerevisiae, the roundworm Caenorhabditis elegans, the fruit fly Drosophila melanogaster, the frog Xenopus laevis, and the zebrafish Danio rerio have made important contributions to our understanding of cancer; however, the laboratory mouse Mus musculus remains still the model of election to design specific therapies and evaluate their efficacy prior to clinical trials.(1)
Scientists have now access to a growing range of preclinical mice, each with their specific strengths and limitations.(3) Here, we briefly review the most relevant models used in preclinical cancer research.
Cell line transplantation models
Cell line transplantation models represent the most commonly used mouse models in onco-immunology. They consist of murine or human cancer cell lines, injected either subcutaneously, orthotopically (to mimic their evolution in a physiological environment), or systemically (to monitor their metastatic spread) in immunocompetent mice.(4) These models are useful to study the pathophysiological relevance of in vivo tumor initiation, and for preclinical drug testing.(5)For example, transplantation models have provided important insights into drug resistance mechanisms and novel combination therapies in colorectal cancer.(6) However, such models do not mirror the intra- and inter-tumor heterogeneity of human cancers1 due to the genetic homogeneity acquired by cell lines through repeated in vitropassages, and are therefore poor predictors of therapy responses.(7)
Patient-derived xenografts
Patient-derived xenografts (PDXs) are established by transplanting fresh human tumor biopsies in immunodeficient mice.(9) Unlike cell line transplantation models, PDXs preserve intra- and inter-tumor heterogeneity as observed in cancer patients, and provide clinically valuable data in various tumors, including colorectal cancer, breast cancer, non-small-cell lung cancer, and prostate cancer.(10) As PDXs are engrafted in immunodeficient mice, they lack a normal adaptive immune system: for this reason, the use of these models is typically restricted to chimeric antigen receptor (CAR)-T and engineered T cell therapy studies.(10)
Genetically engineered mouse models
Genetically engineered mouse models (GEMMs) are sophisticated immunocompetent mice harboring constitutive or inducible mutation(s) that lead to tumor development.(5) Such models have provided the scientific community with important insights supporting the immunosurveillance theory, “a natural physiologic function” that allows “recognition and destruction of transformed cells before they grow into tumors, and kill tumors after they are formed.”(11, 12) GEMMs closely recapitulate human cancer in terms of genetic composition and crosstalk between tumor cells, stroma, and tumor microenvironment; as such, they are useful to identify tumor-initiating and tumor-promoting events, and are therefore of great importance to unveil the complex mechanisms underlying cancer biology.(13) However, GEMMs do have some limitations: first, the generation and validation of these models is laborious and expensive; second, these models may possess unpredicted mutations (namely “off-target effects”) caused by the intrinsic properties of the methods used to develop them (e.g., CRISPR/Cas9); third, these models bear biallelic mutations in the target site, and may therefore give rise to embryo lethal phenotypes.(14, 15)
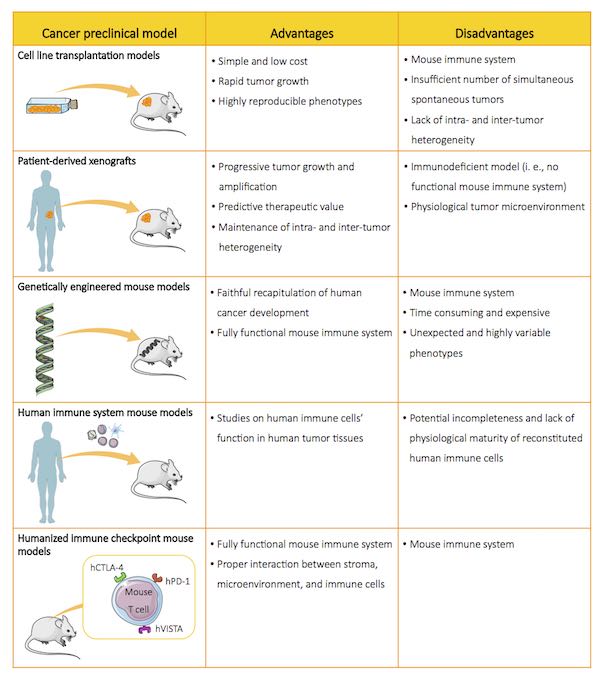
Human immune system mouse models
Immunocompetent mice have been widely used in biomedical research, where they represent effective tools to analyze immune responses directed against engrafted allogeneic tissues. However, critical differences in the genetics and immune systems of mice and humans have precluded certain studies, notably those aiming at assessing drug efficacy. This “gap” has been filled by immunodeficient mice reconstituted with human immune system (HIS).(10) These models have dramatically improved our understanding of the function of the human immune system, and contributed to the study of the complex interactions between myeloid cells, antigen-presenting cells and T cells in reconstituted tumor microenvironments.(16) This has led to the development of novel therapeutics, and the efficacy assessment of immunotherapies prior to translation into the clinic.(3) However, HIS models also have some limitations, including a limited lifespan and incomplete human immune function (e.g., lack of B cell immunoglobulin G responses, underdeveloped lymphoid organs): it is extremely important that these issues are carefully taken into account in the interpretation of the experimental results.(16)
Humanized immune-checkpoint mouse models
Humanized immune-checkpoint (ICP) models are generated by inserting chimeric (i.e., murine and human) ICPs within murine ICP loci.(17) In vivo studies conducted in these animals have been instrumental in assessing the efficacy of immuno-oncology compounds directed against ICP, and in developing new immunotherapies for solid cancers such as metastatic melanomas, non-small-cell lung carcinomas and liver cancer.(13, 16) Moreover, ICP models represent powerful tools for studying how compounds modulate immune cell response and/or stroma cells in a physiological microenvironment.(4) However, these mice possess murine immune systems and therefore fail to recapitulate the potential of individual ICP pathways in regulating T cells and, more generally, immune responses.(16)
Recent technological advances have led to the generation of a wide range of new experimental preclinical models; however, many novel oncology drugs have failed to pass phase II programs.(18) This can be attributed, at least in part, to the misleading interpretations of results obtained in models that do not necessarily best answer specific immunological questions.(2) Accordingly, there is an urgent need to develop a line of sight to the clinic at the very early steps of drug discovery projects, as this will help to select appropriate preclinical models, thereby more efficiently translating preclinical research into successful clinical trials.
Part 2: BRGSF, a new immunodeficient model for immuno-oncology studies
1Cancer heterogeneity refers to the existence of subpopulations of cells that display cellular, genetic, and epigenetic variations within a primary tumor and its metastases (intra-tumor heterogeneity), and between tumors of the same histopathological subtype (inter-tumor heterogeneity).(8)
References:
- Galuschka, Claudia et al. “Models in Translational Oncology: A Public Resource Database for Preclinical Cancer Research.” Cancer Research 77 (2017): 2557. doi: 10.1158/0008-5472.CAN-16-3099.
- Bedognetti, Davide et al. “Toward a comprehensive view of cancer immune responsiveness: a synopsis from the SITC workshop.” Journal for Immunotherapy of Cancer 7 (2019): 131. doi: 10.1186/s40425-019-0602-4.
- Chen, Qingfeng et al. “Cancer immunotherapies and humanized mouse drug testing platforms.” Translational Oncology 12 (2019): 987. doi: 10.1016/j.tranon.2019.04.020.
- Zitvogel, Laurence et al. “Mouse models in oncoimmunology.” Nature Reviews Cancer 16 (2016): 759. doi: 10.1038/nrc.2016.91.
- Kersten, Kelly et al. “Genetically engineered mouse models in oncology research and cancer medicine.” EMBO Molecular Medicine 9 (2017): 137. doi: 10.15252/emmm.201606857
- Morris, Van K. “Systemic therapy in BRAF V600E-mutant metastatic colorectal cancer: recent advances and future strategies.” Current Colorectal Cancer Reports 15 (2019): 53. doi: 10.1007/s11888-019-00429-z.
- Day, Chi-Ping et al. “Preclinical mouse cancer models: a maze of opportunities and challenges.” Cell 163 (2015): 39. doi: 10.1016/j.cell.2015.08.068.
- Stanta, Giorgio, and Serena Bonin. “Overview on Clinical Relevance of Intra-Tumor Heterogeneity.” Frontiers in Medicine 5 (2018): 85. doi: 10.3389/fmed.2018.00085.
- Annibali, Daniela et al. “Development of patient-derived tumor xenograft models.” (2019) In: Fendt S. M., Lunt S. (eds) Metabolic Signaling. Methods in Molecular Biology, vol. 1862. Humana Press, New York, NY. doi: 10.1007/978-1-4939-8769-6_15.
- Saito, Ryoichi et al. “Faithful preclinical mouse models for better translation to bedside in the field of immuno‑oncology.” International Journal of Clinical Oncology (2019). doi: 10.1007/s10147-019-01520-z.
- Zitvogel, Laurence et al. “Mouse models in oncoimmunology.” Nature Reviews Cancer 16 (2016): 759. doi: 10.1038/nrc.2016.91.
- Actor, Jeffrey K. “Chapter 8 – Immunomodulation.” (2012) In: Elsevier's Integrated Review, Immunology and Microbiology. Elsevier doi: 10.1016/B978-0-323-07447-6.00008-9.
- Kalamara, Angeliki et al. “How to find the right drug for each patient? Advances and challenges in pharmacogenomics.” Current Opinion in Systems Biology 10 (2018): 53. doi: doi: 10.1007/978-1-4939-8769-6_15.
- Lee, Ho. “Genetically engineered mouse models for drug development and preclinical trials.” Biomolecules & Therapeutics vol. 22,4 (2014): 267-74. doi: 10.4062/biomolther.2014.074.
- Xu, Cong et al. “Patient-derived xenograft mouse models: A high fidelity tool for individualized medicine.” Oncology Letters vol. 17,1 (2019): 3-10. doi: 10.3892/ol.2018.9583.
- Allen, Todd M. et al. “Humanized immune system mouse models: progress, challenges and opportunities.” Nature Immunology 20 (2019): 770. doi: 10.1038/s41590-019-0416-z.
- Martin, Gaëlle. “Novel humanized CTLA-4 mouse model for assessment of efficacy of biologics.” Tumour Models London Summit, United Kingdom. December 4–6, 2019. Poster presentation.
- Ireson, Christopher R. et al. “The role of mouse tumour models in the discovery and development of anticancer drugs.” British Journal of Cancer 121 (2019): 101. doi: 10.1038/s41416-019-0495-5.
Related products
Catalogue product
Customized product
Scientific excellence
From model design to experimental results
Tailor-made solutions adapted to scientific questions
Robust validation data on catalog models
Comprehensive dataset package
Generated with biopharma partners and in-houseCustomer care
Scientific follow-up and advice along the project
Collaborative approach for problem solving and development of innovative models
Easy and fast access to models
Breeding facilities in US and Europe
Certified health status from professional breeders


